Neuroimaging Fear Conditioning
According to neuroimaging findings, fear conditioning’s CS exposure has been significantly linked with increased autonomic response in skin conductance and activations in the amygdala (Cheng, Knight, Smith, Stein, & Helmstetter, 2003; Morris, Buchel, & Dolan, 2001). The trace interval delay periods, i.e. rest periods between US-CS pairings, have been associated with temporally distinct activations in the hippocampus and in the in the anterior midcingulate cortex (aMCC), a structure associated with declared emotion of anxiety (Buchel, Dolan, Armony, & Friston, 1999; Knight, Nguyen, & Bandettini, 2004a). These activations have also been associated with deactivations in more ventral portions of pACC that interestingly have been associated with other emotions of sadness or happiness, a sense of self-reference, and the ability for emotional perspective taking (Hynes, Baird, & Grafton, 2006; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004; Northoff, 2005; Vogt, Berger, & Derbyshire, 2003). Imaging findings reference temporal sequencing of activations during fear conditioning.
To enhance the understanding of regional dissociative functions on conditioning Bechara and colleagues (Bechara, Tranel, Damasio, Adolphs, Rockland, & Damasio, 1995) studied the impact of respective human lesions. They found that a lesion of the amygdala impaired a patient’s acquisition of conditioned autonomic responses of increased skin conductance (Glascher & Adolphs, 2003) to a startling sound but spared the ability to acquire and verbally report task-related declarative facts about task-related stimulus-context relationships (Bechara,et al. 1995). A hippocampal lesion on the other hand impaired a patient’s ability to learn declarative facts, but had no effect on acquisition of the conditioned autonomic response. These findings indicate that the amygdala processes emotional material via its interaction with structures tied to autonomic activity; the hippocampus is involved in the ability for gaining and reporting declarative knowledge about stimulus-context relationships.
In summary, these studies suggest a role for the hippocampus in supporting the processing of fear-related contexts and a role for the amygdala in modulating hippocampal declarative function. They also reflect a functional dissociation between both structures during fear conditioning, whereby the amygdala is involved in CS-US unpairing and linkage to autonomic centers and arousal and the hippocampus in declarative aspects.
References
Bechara, A., Tranel, D., Damasio, H., Adolphs, R., Rockland, C., & Damasio, A.R. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269(5227), 1115-8.
Buchel, C., Dolan, R.J., Armony, J.L., & Friston, K.J. (1999). Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. Journal of Neuroscience, 19(24), 10869-10876.
Cheng, D.T., Knight, D.C., Smith, C.N., Stein, E.A., & Helmstetter, F.J. (2003). Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behavioral Neuroscience, 117(1), 3-10.
Glascher, J., & Adolphs, R. (2003). Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience, 23(32), 10274-82.
Hynes, C.S., Baird, A.A., Grafton, S.T. (2006). Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia, 44(3), 374-383.
Knight, D.C., Nguyen, H.T., & Bandettini, P.A. (2004). Expression of conditioned fear with and without awareness. Proceedings of the National Academy of Sciences U.S.A., 100(25), 15280-3.
Macrae, C.N., Moran, J.M., Heatherton, T.F., Banfield, J.F., & Kelley, W.M. (2004). Medial prefrontal activity predicts memory for self. Cerebral Cortex, 14(6), 647-654.
Morris, J.S., Buchel, C., & Dolan, R.J. (2001). Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage, 13, 1044-1052.
Northoff, G. (2005). Is emotion regulation self-regulation? Trends in Cognitive Sciences, 9(9), 408-409.
Vogt, B.A., Berger, G.R., & Derbyshire, S.W. (2003). Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience, 18(11), 3134-3144. pdf
Extinction & its Role in Consolidation
There are two ways of inhibiting responses of increased acute autonomic arousal and fear, extinguishing them or suppressing them (Cammarota, Barros, Vianna, Bevilaqua, Coitinho, Szapiro et al., 2004). Both are linked with two different processes. Extinction is linked with the concluding of conditioning processes allowing expression of another type of learning about a previous fear inducing CS (Brooks & Bouton, 1993; Pavlov, 1927). Suppression is linked with forgetting and incomplete processing of fear-related material. These processes will be elaborated on and differentiated in subsequent sections.
The previous findings are supported in human neuroimaging research. During an event-related paradigm Phelps and colleagues (2004) observed that fear conditioning learning (i.e. images of blue squares (CS) while being paired with an aversive US, mild shock to the wrist) and extinction (unpairing CS-US) were characterized by temporally sequenced phases suggestive of consolidation processes (Phelps, Delgado, Nearing, & LeDoux, 2004). These phases were associated by activations of different brain regions. Accordingly the acquisition phase (i.e. during the delays after pairing trials) was associated with initial reactive cue dependent autonomic arousal (i.e. SCR-skin conductance response) and significant prefrontal activity in the anterior (rostral) portions of the midcingulate (MCC), the anterior insular cortex (INS) and caudate nucleus (Buchel, Dolan, Armony & Friston, 1999). These structures have previously been associated with autonomic arousal (Augstine, 1996; Critchley, Mathias, Josephs, O’Doherty, Zanini, Dewar et al., 2003; Kuniecki, Urbanic, Sobiecka, Kozub, & Binder, 2003), pain perception (Frot & Mauguiere, 2003; Leone, Proietti Cecchini, Mea, Tullo, Curone, & Bussone, 2006; Rainville, Duncan, Price, Carrier, & Bushnell, 1997), the emotion of fear (Damasio, Grabowski, Bechara, Damasio, Ponto et al., 2000; Vogt, Berger, & Derbyshire, 2003), and event-related fear conditioning (Buchel et al., 1999) in the human.
During the next phase of fear conditioning or extinction (US-CS unpairing) subjects were subjected to the CS in absence to the aversive US (wrist shock). This phase was entitled Day 1 Extinction. Task performance showed enhance fear conditioning effects, as the CS alone was able to elicit autonomic arousal reflected in increased skin conductance response (SCR). Reactive activation values to the CS for previously noted midcingulate and insular cortices decreased. Phase activations in these regions coupled with enhanced and sustained autonomic arousal were probably reflective of amygdala-associated brainstem influences (Liddell, Brown, Kemp, Barton, Das, Peduto et al., 2005).
During the next and last testing session and further CS unpairing (Day 2 Extinction), BOLD signals and activations allowed expression of areas that had initially been associated with initial autonomic arousal during pairing US-CS noted above (i.e. the MCC and anterior insular cortex during reactive response) however with the strength of autonomic arousal decreased dramatically. Extinction-induced regional changes negatively correlated with autonomic arousal (Critchley, Tang, Glaser, Butterworth, & Dolan, 2005).
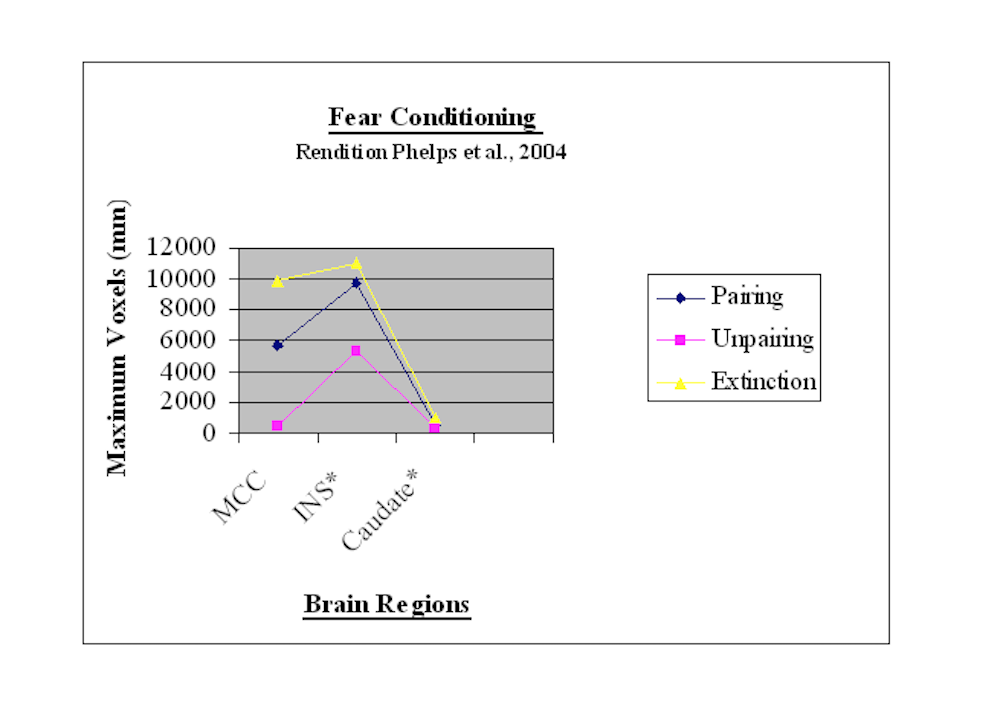
The previous discussion can be represented in the subsequent bar chart.
For BOLD signals and regional activations involving both hemispheres, the single largest value was selected for this chart’s development. Voxel values are reflected vertically on the y-axis. The phases of fear conditioning are noted horizontally on the x-axis. Each bar in the chart represents a different brain region during each of the three phases. For instance the chart reflects that the MCC and insula engage more voxel activations during pairing and extinction but fewer voxels during the unpairing phase
This point graph depicts the BOLD signal in voxels on the y-axis and brain regions on the x-axis within context of the three different phases, i.e. pairing, unpairing, and extinction. The point chart’s findings correspond to those provided for the bar chart.
These findings suggest that there is a temporal sequencing for consolidating fear conditioning learning and extinction. Consolidation processes seem to begin with fear acquisition learning (Phelps et al., 2004). Accordingly the pairing phase is associated with activations in the anterior cingulate and insular cortices as well as caudate nucleus (Buchel et al., 1999). As noted previously plasticity in anterior regions of the medial (and lateral prefrontal) cortex is probably needed for the expression of the next phase (second unpairing phase) of fear-instilling avoidance conditioning (Gabriel, Kubota, Sparenborg, Straube, & Vogt, 1991). This next phase, the unpairing phase, according to the above findings is characterized by reduced expression in the anterior cingulate and insular cortices.
As noted earlier the animal research has referenced involvement of other brain regions during fear conditioning processes. Their findings suggest that the amygdala mediates CS-US pairing. The posterior cingulate cortex (PCC) (Yonelinas, Otten, Shaw, & Rugg, 2005) and parahippocampus of the hippocampal formation (Maratos, Dolan, Morris, Henson, & Rugg, 2001) in the later phases of single session learning have been shown to support cue-specific recallable recognition. This is an important distinction for understanding of consolidation processes, as “cue dependent” recallable recognition is a precursor for later cue independent recallable retrieval. Interestingly, according to the animal research, initial involvement of the posterior cingulate cortex and hippocampal region signals the organism’s immediate response to removing and unpairing the initial US (Gabriel et al., 1991; Kang & Gabriel, 1998). In another neuroimaging study PCC rCBF activity during the unpairing or second phase of fear conditioning (Doronbekov, Tokunaga, Ikejiri, Kazui, Hatta, Masaki et al., 2005) may be suggestive of either its regional dependent consolidation involvement in CS cue dependent recognition or its initial CS unpairing or both. These findings suggest a shift from one stage of consolidation to another with US removal. Finally, there is an extinction phase that returns neural activity back to anterior structures but without apparent autonomic arousal. These regions also support cue-nonspecific and the hippocampal-independent recallable retrieval of autobiographical memory over time (Maguire, Henson, Mummery, & Frith, 2001). As noted earlier in this web site this supports hippocampal lesioning retrograde amnesia studies, which suggest that an intact prefrontal cortex not an intact hippocampus is needed for remote memory retrieval.
References
Anderson, M.C., Ochsner, K.N., et al., (2004). Neural systems underlying the suppression of unwanted memories. Science, 303(5655), 232-235.
Augustine, J.R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research-Brain Research Review, 22(3), 229-244.
Brooks, D.C., & Bouton, M.E. (1993). A retrieval cue for extinction attenuates spontaneous recovery. Journal of Experimental Psychology: Animal and Behavior Processes, 19(1), 77-89.
Buchel, C., Dolan, R.J., Armony, J.L., &Friston, K.J. (1999). Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. Journal of Neuroscience, 19(24), 10869-10876.
Cammarota, M., Barros, D.M., Vianna, M.R., Bevilaqua, L.R., Coitinho, A., Szapiro, G., Izquierdo, L., Medina, J.H., & Izquierdo, I. (2004). The transition from memory retrieval to extinction. Annals of the Brazilian Academy of Sciences, 76(3), 573-582.
Critchley, H.D., Mathias, C.J., Josephs, O., O’Doherty, J., Zanini, S., Dewar, B.K., Cipolotti, L., Shallice, T., & Dolan, R.J. (2003). Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain, 126(Pt 10), 2139-2152.
Critchley, H.D., Tang, J., Glaser, D., Butterworth, B., & Dolan, R.J. (2005). Anterior cingulate activity during error and autonomic response. Neuroimage, 27(4), 885-895.
Damasio, A.R., Grabowski, T.J., Bechara, A., Damasio, H., Ponto, L.L., Parvizi, J., & Hichwa, R.D. (2000). Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience, 3(10), 1049-1056.
Doronbekov, T.K., Tokunaga, H., Ikejiri, Y., Kazui, H., Hatta, N., Masaki, Y., Ogino, A., Miyoshi, N., Oku, N., Nishikawa, T., & Takeda, M. (2005). Neural basis of fear conditioning induced by video clip: positron emission tomography study. Psychiatry and Clinical Neurosciences, 59(2), 155-162.
Frot, M., & Mauguiere, F. (2003). Dual representation of pain in the operculo-insular cortex in humans. Brain, 126(Pt 2), 438-450.
Gabriel, M., Kubota, Y., Sparenborg, S., Straube, K., & Vogt, B.A. (1991). Effects of cingulate cortical lesions on avoidance learning and training-induced unit activity in rabbits. Experimental Brain Research, 86(3), 585-600.
Kang, E., & Gabriel, M. (1998). Hippocampal modulation of cingulo-thalamic neuronal activity discriminative avoidance learning in rabbits. Hippocampus, 8(5), 491-510.
Kuniecki, M., Urbanik, A., Sobiecka, B., Kozub, J., & Binder, M. (2003). Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiologiae Experimentalis, 63(1), 39-48.
Leone, M., Proietti Cecchini, A., Mea, E., Tullo, V., Curone, M., & Bussone, G. (2006). Neuroimaging and pain: a window on the autonomic nervous system. Neurological Science, 27(Supplement 2), S134-137.
Liddell, B.J., Brown, K.J., Kemp, A.H., Barton, M.J., Das, P., Peduto, A., Gordon, E., &
Maguire, E.A., Henson, R.N., Mummery, C.J., & Frith, C.D. (2001). Activity in prefrontal cortex, not hippocampus varies parametrically with increasing remoteness of memories. Neuroreport, 12(3), 441-444.
Maratos, E.J., Dolan, R.J., Morris, J.S., Henson, R.N., & Rugg, M.D. (2001). Neural activity associated with episodic memory for emotional context. Neuropsychologia, 39(9), 910-920.
Ohira, H., Nomura, M., et al., (2006). Association of neural and physiological responses during voluntary emotion suppression. Neuroimage, 29(3), 721-33.
Pavlov, I.P. (1927). Conditioned Reflexes. (Translated by G.V. Anrep) Cambridge: Oxford University Press (Lectures 2, 4, & 5).
Phelps, E.A., Delgado, M.R., Nearing, K.I., & LeDoux, J.E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron, 43(6), 897-905.
Rainville, P., Duncan, G.H., Price, D.D., Carrier, B., & Bushnell, M.C. (1997). Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science, 277(5328), 968-971.
Vogt, B.A., Berger, G.R., & Derbyshire, S.W. (2003). Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience, 18(11), 3134-3144.
Williams, L.M. (2005). A direct brainstem-amygdala-cortical ?alarm’ system for subliminal signals of fear. Neuroimage, 24(1), 235-243. Hippocampal and amygdaloid activations disappeared with concurrent significant decreases in the posterior cingulate cortex.
Wyland, C.L., Kelley, W.M., et al., (2003). Neural correlates of thought suppression. Neuropsychologia, 41(14), 1863-7.
Yonelinas, A.P., Otten, L.J., Shaw, K.N., & Rugg, M.D. (2005). Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience, 25(11), 3002-3008.