Human Social Reward
Rewarding objects encourage and elicit responses for reward expectation and cognitive planning for later expression of approach behaviors seeking reward attainment. Tangible sensory rewards, i.e. those objects that elicit positive sensations, like foods that are sweet, salty or rich to the sense of taste or pleasant touch elicit a positive sense of well-being. Intangible sensory rewards, like melodic, rhythmic, seductive, and soothing sounds, symmetrical and pleasing appearances, or sweet, pungent, and aromatic odors generate positive senses of well-being and the emotion of happiness with attainment and experience.
In addition to tangible and intangible sensory rewards there are also tangible and intangible social rewards. Like tangible sensory rewards, tangible social rewards evoke positive senses of well-being when one receives an object that fosters a positive sense of well-being, like a treasured toy, piece of jewelry, monetary incentive, etc. In contrast intangible social rewards are not physical objects but are elicited during social interactions. Social interactions generate a positive sense of well-being when one feels that one belongs, is accepted by others during social interactions, and is able to experience mastery during tasks or during social interactions. Both tangible and intangible sensory and intangible social rewards are capable of instilling rewarding outcomes of positive senses of well-being and positive emotions like happiness and contentedness. Human beings and animals alike hope, anticipate, and expect to obtain rewards and associated positively rewarding outcomes.
Both tangible and intangible sensory and social rewards can be characterized as being unlearned primary positive reinforcers. They are unlearned because primary positive reinforcers are spontaneously capable (without prior exposure) of motivating approach behaviors for reward attainment. For instance, when hungry, certain types of food (tangible sensory reward), can alleviate hunger pains. Food’s rewarding qualities in this situation center on its abilities for alleviating discomforting hunger pains. Parental or spousal comfort, i.e. a soothing embrace and empathetic tone of voice (tangible and intangible social reward), is perceived as socially rewarding by alleviating distress, discomfort, and pain.
Though primary reinforcers are seemingly unlearned, the environment does play a role in shaping food preferences or other preferences for primary reward. Different cultures early in ontegenetic development likely shape which objects, things, social interactions will later be acceptably rewarding. For instance the familiarity and comfort experienced with a life long vegetarian diet may interfere in beef’s rewarding qualities and abilities for reducing hunger by generating the emotion of disgust with the idea of meat. Pleasant soothing touch may be interpreted as unpleasant. Certain cultures advise mothers not to comfort crying and distressed babies seeking attention and soothing. Other children are exposed to persistent physical child abuse which fosters a sense of aversion with any type of touch. When these children develop into adults, they often resist comforting, soothing, and intimacy to recover from perceived distress. But despite these exceptions it is likely that the environment has a role in shaping the nature of primary reinforcers, which when selected, are perceived as having rewarding qualities.
Both tangible and intangible sensory and social rewards can also assume secondary reinforcer qualities. When a salesman tries to instill in his client a positive sense of well-being when he takes him out to a lavish meal, good drink, a date with a sexy woman, etc., the salesman, is trying to associate himself and the prospective business deal (secondary reinforcer) with many different primary sensory tangible and social intangible reinforcers to cement the deal.
A child’s sense of love and affiliation derived from social interactions with a parent (the primary reinforcing experience) can later be experienced into adulthood within adult intimate relationships (secondary reinforcer). (Future animal research will likely substantiate how the early ontogenetic expression of tender dopamine neurocircuitry in response to early primary social reinforcers likely underlie later dopamine expression in response to secondary social reinforcers.) The relationship between early childhood primary reinforcer classification and later adult secondary reinforcer designation is important for helping us to understand the nature and extent of early childhood influences on later adult social relationships and social interactions. The above can be summarized in the following chart.
Examples of Approach Seeking Socially Rewarding Stimuli
| Primary Social Reinforcers | Secondary Social Reinforcers | |
|---|---|---|
| Intangible | Childhood sources of primary intangible social reinforcers (e.g. parents, siblings, teachers, friends, etc.) offer the child a source for affiliation and belonging, control over outcomes & agency, personal sense of achievement and mastery, social (other) validation of self during social interactions, and positive sense of well-being. | Adult sources of secondary intangible social reinforcers (e.g. spouses, significant other, friends, co-workers, business associates, etc.) offer the adult sources for affiliation and belonging, control over outcomes & agency, personal sense of achievement and mastery, social (other) validation of one's productions, and positive sense of well-being. |
| Tangible | Primary tangible socially rewarding objects are associated with a positive sense of well-being. For example adult tangible social rewards themselves foster greater ease and convenience with their use by removing anxiety-producing impediments and anxiety, e.g. heavy duty washing machine and dryer. Childhood tangible social rewards like toys that stimulate interest, novelty, etc., may be considered tangible social rewards. | Secondary tangible socially reinforcing objects generate the ability for acquiring access to primary tangible and intangible social rewards. Monetary rewards, as secondary reinforcers, offer the ability for acquiring tangible social rewards, like items and objects that foster a positive sense of well-being or intangible rewards that elevate ones social status and the validation of others. Expensive brilliant jewelry fosters senses of both tangible sensory reward of visual beauty and secondary social intangible reward of increased status and validation of others that are associated with this item's purchase price. |
Regionalization of Tangible and Intangible Reward Activity & Its Meaning
According to neuroimaging findings intangible rewards are like tangible sensory rewards in so far as their ability for eliciting access and activating corresponding inner reward neurocircuitry in certain brain regions in the central nervous system, like the cingulate cortex, orbitofrontal cortex, and the ventral striatal’s caudate nucleus and nucleus accumbens. As indicated in the Future Direction page of this web site, this writer has discovered a good deal about the mind and behavior during studies of the cingulate cortex. The following will reflect this knowledge.
As referenced and summarized in the diagram below, tangible sensory rewards (i.e. food and tactile stimulation), tangible social rewards (i.e. monetary), and intangible social reward (i.e. reward derived from social interactions) activate the rostral midcingulate cortex (rMCC) and caudal perigenual anterior cingulate cortex (cpACC) area and track towards the rostral pACC area with increasing reward satiation. Activations traverse in a caudorostral fashion depending on the respective neuroimaging studies task dependent reward state of uncertainty (Bush, Vogt, Holmes, & Dale, 2002; Critchley, Mathias, & Dolan, 2001; Keri, Decety, Roland, Gulyas, & Keri, 2004), certainty (Knutson, Fong, Adams, Varner, & Hommer, 2001; Knutson, Fong, Bennett, Adams, & Hommer, 2003; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005) and reward attainment (Blood & Zatorre, 2001; O’Doherty, Kringelback, Rolls, Hornak, & Andrews, 2001), even under conditions of unexpected and attained reward (Ramnani, Elliott, Athwal, & Passingham, 2004).
However, when expectation for sensory reward is breached and not delivered as expected, a negative sense of well-being is instilled in response to the perceived sense of loss of reward and negative emotion. For instance when desired tangible sensory food reward is withheld the outcome is hunger pains and emotions of disappointment, sadness, frustration, or anger. When water is denied, the outcome is uncomfortable thirst and may lead to emotions of frustration and anger.
Likewise when intangible social reward is breached and not delivered, a negative sense of well-being also sets in response to the perceived sense of loss and negative emotion. However, typically, breaches in intangible social reward, elicit greater diversity of emotion, depending upon the nature and intensity of motivation underlying social reward expectation. For instance, rejection of attempts for affiliation and social rewarding effects for attention, company, and companionship may generate a negative sense of well-being, visceral sensation of emotional pain in the upper gastrointestinal tract or in the (heart region of the) chest. Blocking attempts and expectations for control over outcomes, and not getting what one wants, may elicit a negative sense of well-being, visceral sensation of heaviness in the chest, increased blood pressure, flushed face and initial feelings of helplessness and later feelings of anger, frustration, and further persistence for social goals for control over outcomes. The inability for meeting ones task-dependent achievement expectations may instill a negative sense of well-being, visceral sensation of emotional pain in the upper gastrointestinal tract or in the (heart region of the) chest, and feelings of self-disappointment, helplessness with ones short-comings, and sadness with loss of personal integrity.
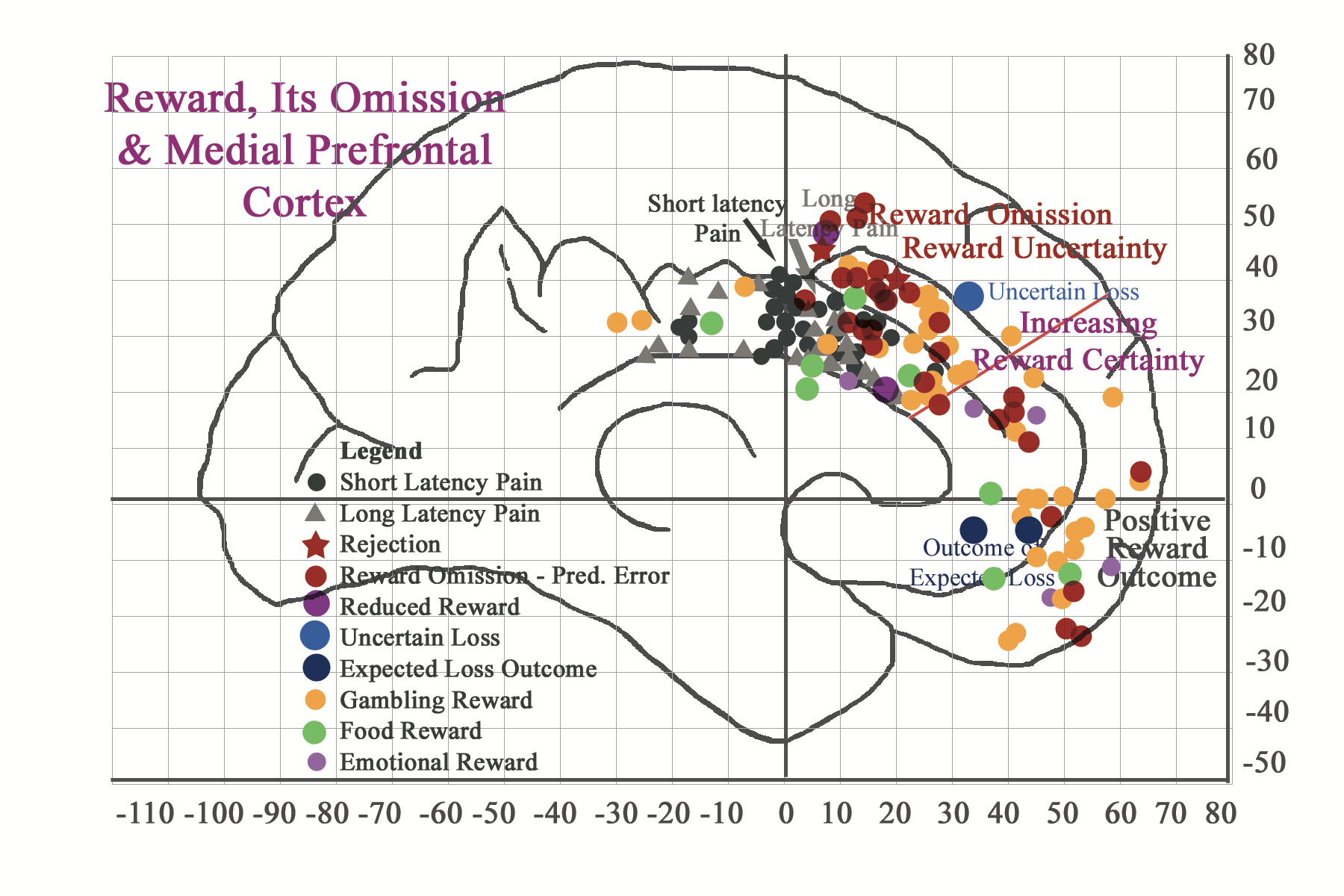
According to neuroimaging findings, breaches in reward expectation, i.e. reward omission of expected sensory tangible rewards and intangible rewards, as with rejection from social inclusion or with violation of correct task dependent decision-making, can instill a good deal of perceived emotional pain. This sense of emotional pain is also reflected in neuroimaging findings for reward omission. Failure of social (and culturally determined) tangible task-dependent reward delivery can also be considered as a negative reward prediction error, i.e. reward was not delivered as predicted (Tobler, O’Doherty, Dolan, & Schulz, 2006; Ullsperger & von Cramon, 2003). Interestingly, neuroimaging citations relating to errors in reward prediction (O’Doherty, Dayan, Friston, Critchley, & Dolan, 2003) also correspond to regionalized long latency (>/= 15 sec) pain expression (Vogt, Berger, & Derbyshire, 2003) and reward monitoring (Bush et al., 2002) in the rostral midcingulate cortex (MCC) region , respectively. Omitted intangible social rewards, like those involving rejection from social inclusion or social violation of correct task dependent strategic decision-making, also generate a good deal of perceived emotional pain. According to Lieberman and colleagues (2003) this sense of emotional pain is also reflected in neuroimaging findings of rejection (reflected as a star in the picture below). According to respective neuroimaging citations emotional reactions to task-dependent simulations of social alienation congregate around the long latency pain region and reward prediction error area of the rostral midcingulate cortex. These findings confirm the presence of shared regionalization of both physical and emotional pain. They also suggest that painful parameters may also be associated with breaches in expectations of reward-associated positive sense of well being.
And if I put both concepts of reward attainment and reward omission in one illustration, I get the following. Notice that reward expectation and uncertainty centers in the caudal perigenual rregion of the cingulate cortex and as it becomes more certan it congregates in more anterior and ventral regions.
We can conclude from the above analysis that tangible and intangible reward processing in the brain (and also in the mind) is really undifferentiated. We may also conclude that as reward expectation is fulfilled, the signal of the expected outcome travels caudo-rostrally and concludes ventro-rostrally, in response to the perceived match with reward attainment. When reward expectation is not fulfilled, the signal remains in caudal regions, which are associated with and characterized by experiences of uncertainty and long latency pain. One may conclude that the mismatch for an expected reward outcome or undelivered reward serves to disrupt reward pathways and interferes in the brain’s (and the mind’s) normal processing of well-being. Future sections in the Future Direction section will elaborate on these concepts and others.
References
Blood, A.J. & Zatorre, R.J. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences, USA, 98(20), 11818-11823.
Bush, G., Vogt, B.A., Holmes, J., Dale, A.M. Greve, D., Jenike, M.A., Rosen, B.R. (2002). Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences, USA, 99(1), 523-528.
Critchley, H.D., Mathias, C.J., Dolan, R.J. (2001). Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron, 29(2), 537-545.
Eisenberger, N.I., Lieberman, M.D., Williams, K.D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302, 290-292.
Keri, S., Decety, J., Roland, P.E., Gulyas, B. (2004). Feature uncertainty activates anterior cingulate cortex. Human Brain Mapping, 21(1), 26-33.
Knutson, B., Taylor, J., Kaufman, M., Peterson, R., Glover, G. (2005). Distributed neural representation of expected value. Journal of Neuroscience, 25(19), 4806-4812.
Knutson, B., Fong, G.W., Bennett, S.M., Adams, C.M., Hommer, D. (2003). A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage, 18(2), 263-272.
Knutson, B., Fong, G.W., Adams, C.M., Varner, J.L., Hommer, D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport, 12(17), 3683-3687.
O’Doherty, J., Kringelback, M.L., Rolls, E.T., Hornak, J., Andrews, C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4(1), 95-102.
O’Doherty, J.P., Dayan, P., Friston, K., Critchley, H., Dolan, R.J. (2003). Temporal difference models and reward-related learning in the human brain. Neuron, 38(2), 329-337.
Ramnani, N., Elliott, R., Athwal, B.S., Passingham, R.E. (2004). Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage, 23(3), 777-786.
Tobler, P.N., O’Doherty, J.P., Dolan, R.J., Schultz, W. (2006). Human neural learning depends on reward prediction errors in the blocking design. Journal of Neurophysiology, 95(1): 301-310.
Ullsperger, M., & von Cramon, D.Y. (2003). Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. Journal of Neuroscience, 23(10), 4308-4314.